Atomic Mass of Mercury Atomic mass of Mercury is 200.59 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu). ››More information on molar mass and molecular weight. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Mercury, isotope of mass 203. 203-Mercury vapour (203Hg)Mercury. Mercury have boiling and melting points of 356.9 C and -38.87, respectively. It has atomic number of 80 and a molecular weight of 200.59 and belong to the Group 12 (Zinc group, II b) of the periodic table.
 Article
Article - Basic astronomical data
- Character of the surface
- Caloris
- Origin and evolution
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!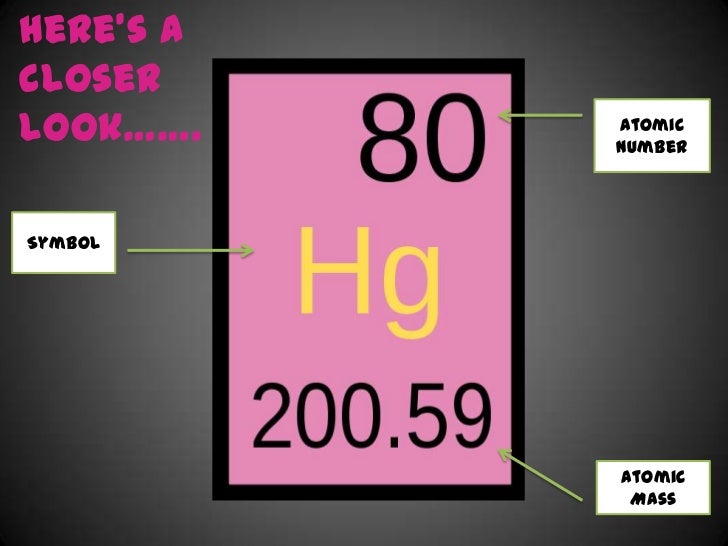
Font natasya font extra. Mercury, the innermost planet of the solar system and the eighth in size and mass. Its closeness to the Sun and its smallness make it the most elusive of the planets visible to the unaided eye. Because its rising or setting is always within about two hours of the Sun’s, it is never observable when the sky is fully dark. Mercury is designated by the symbol ☿.
The difficulty in seeing it notwithstanding, Mercury was known at least by Sumerian times, some 5,000 years ago. In Classical Greece it was called Apollo when it appeared as a morning star just before sunrise and Hermes, the Greek equivalent of the Roman god Mercury, when it appeared as an evening star just after sunset. Hermes was the swift messenger of the gods, and the planet’s name is thus likely a reference to its rapid motions relative to other objects in the sky. Even in more recent eras, many sky observers passed their entire lifetimes without ever seeing Mercury. It is reputed that Nicolaus Copernicus, whose heliocentric model of the heavens in the 16th century explained why Mercury and Venus always appear in close proximity to the Sun, expressed a deathbed regret that he had never set eyes on the planet Mercury himself.
Hg Atomic Mass
Until the last part of the 20th century, Mercury was one of the least-understood planets, and even now the shortage of information about it leaves many basic questions unsettled. Indeed, the length of its day was not determined until the 1960s, and Mercury’s nearness to the Sun gave scientists bound to Earth many observational hurdles, which were overcome only by the Messenger (Mercury Surface, Space Environment, Geochemistry, and Ranging) probe. Messenger was launched in 2004, flew past the planet twice in 2008 and once in 2009, and settled into orbit in 2011. It mapped the entire surface of Mercury before crashing into the planet in 2015. Mercury’s proximity to the Sun has also been exploited to confirm predictions made by relativity theory about the way gravity affects space and time.
| Planetary data for Mercury | |
|---|---|
| *Time required for the planet to return to the same position in the sky relative to the Sun as seen from Earth. | |
| mean distance from Sun | 57,909,227 km (0.39 AU) |
| eccentricity of orbit | 0.2056 |
| inclination of orbit to ecliptic | 7.0° |
| Mercurian year (sidereal period of revolution) | 87.97 Earth days |
| maximum visual magnitude | −1.9 |
| mean synodic period* | 116 Earth days |
| mean orbital velocity | 47.36 km/sec |
| radius (mean) | 2,439.7 km |
| surface area | 74,797,000 km2 |
| mass | 3.30 × 1023 kg |
| mean density | 5.43 g/cm3 |
| mean surface gravity | 370 cm/sec2 |
| escape velocity | 4.25 km/sec |
| rotation period (Mercurian sidereal day) | 58.646 Earth days |
| Mercurian mean solar day | 175.9 Earth days |
| inclination of equator to orbit | 0° |
| magnetic field strength | 0.003 gauss |
| mean surface temperature | 440 K (332 °F, 167 °C) |
| surface temperature extremes | |
| 700 K (800 °F, 430 °C); | |
| 90 K (−300 °F, −180 °C) | |
| typical surface pressure | about 10−15 bar |
| number of known moons | none |

Mercury Atomic Symbol
At first glance the surface of the planet looks similar to the cratered terrain of the Moon, an impression reinforced by the roughly comparable size of the two bodies. Mercury is far denser, however, having a metallic core that takes up about 61 percent of its volume (compared with 4 percent for the Moon and 16 percent for Earth). Moreover, its surface shows significant differences from lunar terrain, including a lack of the massive dark-coloured lava flows known as maria and the presence of buckles and scarps that suggest Mercury is shrinking.
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
Mercury Element Atomic Mass
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
Atomic Mass Of Mercury
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
